Abstract
Background: Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma worldwide. Although most patients with DLBCL respond to first-line chemoimmunotherapy with R-CHOP, approximately 40% of patients will have relapsed or refractory disease. Currently, the second-line therapeutic regimen is chemoimmunotherapy followed by consolidative autologous hematopoietic stem cell transplantation (AHCT) for relapsed/refractory DLBCL (R/R DLBCL). However, the prognosis has remained poor. (Sawalha. J Pers Med. 2021). Thus, discovering effective treatment regimens for patients with R/R DLBCL has become extremely crucial. Recently, a combination regimen of lenalidomide, anti-programmed cell death protein-1 (PD-1) antibody, and rituximab demonstrated a good efficacy with an overall response rate (ORR) of 40% in R/R DLBCL (Sethi et al. Blood. 2019). Activation of the Bruton's tyrosine kinase (BTK)-mediated B-cell receptor (BCR) signaling pathway is a hallmark of DLBCL (Kapoor et al. Cell Death Dis. 2019). Orelabrutinib, a novel and potent irreversible BTK inhibitor, has shown encouraging efficacy with an ORR of 61.5% in combination with a new generation anti-CD20 antibody MIL62 in R/R DLBCL (Xu et al. Blood. 2020; Thus, we assessed the efficacy and safety of lenalidomide, anti-PD-1 antibody combined with orelabrutinib or rituximab in R/R DLBCL.
Methods: This was a prospective, multicenter, single-arm, open-label, phase II study (ChiCTR2200056256). Patients aged 18 to 75 years were eligible if they had histologically confirmed R/R DLBCL, an Eastern Cooperative Oncology Group performance status of 0-2, adequate organ and bone marrow function, and survival time ≥ 6 months. Eligible patients received a combination regimen (21-day/cycle) of lenalidomide (10-25 mg, days 1-10, QD, oral), Tislelizumab (200 mg, day 1, intravenous), and orelabrutinib (150 mg, QD, oral) or rituximab (375 mg/m2, day 1, intravenous). The primary endpoint was ORR. Secondary endpoints included complete response (CR), progression-free survival (PFS), duration of response (DOR), time to response (TTR) and adverse events (AEs). Efficacy was assessed using Lymphoma Response to Immunomodulatory therapy Criteria (LYRIC, 2016). AEs were assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) v 4.0.
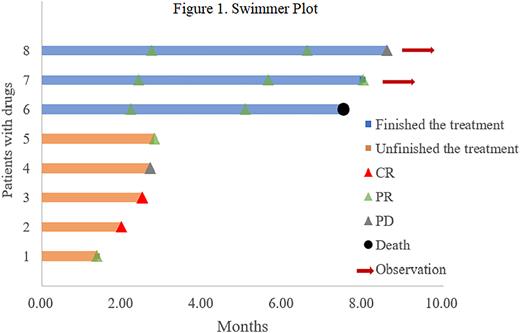
Results: Between August 2021 and March 2022, 9 patients (6 males; median age, 73 years, range 65-76) were enrolled. Among them, 8 patients were non-germinal center B-cell-like and 1 was unknown. Most patients had comorbidities (n=8, 88.9%), an Ann Arbor stage of III or IV (n=4, 44.4%), International Prognostic Index (IPI) ≥ 3 (n=4, 44.4%). All patients received lenalidomide, anti-PD-1 antibody combined with orelabrutinib regimen, and the median duration of treatment was 5 cycles (range 3-6). Among them, 4 patients (44.4%) received a second-line therapy, and 5 patients (55.5%) received ≥ third-line therapy. Before enrollment, 3 patients attained a CR as their best response during the previous therapies. Eight patients were included in the efficacy analysis. The ORR, CR and median TTR were 87.5%, 25.0%, and 2.45 months (range 1.97-2.73), respectively. Among them, 3 patients completed 6 cycles of therapy with ORR of 100% (Figure 1). The PFS and DOR were not completely mature due to the short follow-up period at the end of follow-up. Eight (80%) patients reported AEs. The most common hematological AEs were anemia (77.8%), platelet count reduced (55.6%), neutrophil count decreased (33.3%) and white blood cell count decreased (22.2%). Three patients (33.3%) discontinued treatment due to AEs (atrioventricular block, n=1; rash, n=2). In addition, the most common AEs were grade 1 or 2, and no grade 4 AEs. One patient died due to electrolyte disturbances and depression.
Conclusion: Lenalidomide, anti-PD-1 antibody combined with orelabrutinib or rituximab regimen is effective and well-tolerated for patients with R/R DLBCL in this phase II study. This study provides a potential novel strategy for the treatment of R/R DLBCL.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal